Pain and Its Management
Pain is an unpleasant sensation that can negatively affect all areas of a person's life, including comfort, thought, sleep, emotion, and normal daily activity. Chronic, untreated pain can disturb quality of life and social functioning and disrupt employment. The pain sensation results from complex phenomena that involve physical perception as well as the emotional reaction to the perception. Physiologic variables (e.g., tissue injury) and psychological variables (e.g., anxiety) influence a person's reaction to pain. Ascending neural pathways transfer electrochemical pain signals from the periphery to the central cortex where perception occurs, whereas descending pathways may attenuate or modulate signaling back to the site where the pain is felt. The pain sensation is therefore the net effect of complicated interactions of ascending and descending neural pathways with biochemical and electrochemical processes.
Pain is categorized according to its cause, location, duration, and clinical features. The most simplistic categorization involves differentiating brief-duration (acute) pain from long-lasting (chronic) pain syndromes. Acute pain serves a useful purpose of alerting an individual to an injury and initiating a reflex withdrawal from a noxious or offensive stimulus. In contrast, persistent (chronic) pain serves no biologic protective purpose and can cause undue stress and suffering. Pain was formerly believed to be merely a symptom and not a diagnosis. Recent advances in molecular biology and the understanding of neural mechanisms have demonstrated, however, that chronic pain can lead to long-lasting changes in the nervous system, a phenomenon known as neural plasticity. This concept is particularly important to the understanding of chronic pain because it helps explain difficulties observed in treating various painful conditions. Although initially considered static, pain processes are now realized to be plastic. Such changes therefore define a disease or process that induces physiologic change in the body. Regardless of its origin, however, pain requires a thorough evaluation to determine the underlying cause. By definition, pain is a subjective experience. Therefore, the patient is the only person who can best describe the intensity and character of pain. Pain is whatever the experiencing person says it is, existing wherever he or she says it does.
Because pain is a variable and personal experience, it is difficult to describe completely and measure objectively. The clinician, therefore, must guard against personal biases, which can interfere with treatment. It is important also to rely on tools, such as pain scales, to communicate with patients and understand the extent of their pain. Such tools allow clinicians to objectively measure the clinical results of their interventions. Most often with chronic painful conditions, total pain elimination is not a realistic goal, owing to the nervous system changes described above. Instead, a more attainable objective is reducing pain to a tolerable predetermined level as agreed on by consensus of the patient and clinician. A person with chronic pain should expect to achieve pain reduction with the ultimate objective of increasing daily function (e.g., activities of daily living) and minimizing suffering.
Mechanisms of Pain
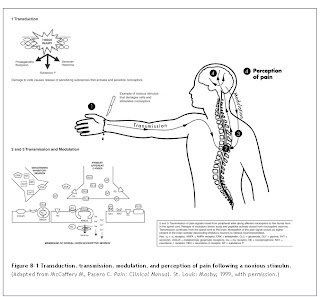
Transduction, Transmission, Modulation, and Perception
Pain sensation involves a series of complex interactions between peripheral nerves and the central nervous system (CNS). This process is modulated by excitatory and inhibitory neurotransmitters released in response to stimuli. Such stimuli can be physical, psychological, or both. An example of a physical stimulus is a burn or cut to the skin. In a short time, local reactions occur in the damaged area that initiate the release of chemical mediators involved in inflammation. This is followed by sensitization of the nerve endings, which ultimately send signals to the sensory cortex of the brain. Nociception, or the sensation of pain, is composed of four basic processes: transduction, transmission, modulation, and perception (Fig. 8-1).1
Transduction is the process by which noxious stimuli are translated into electrical signals at peripheral receptor sites. This begins when nociceptors (free nerve endings located throughout the skin, muscle, and viscera) are exposed to a sufficient quantity of mechanical, chemical, or thermal noxious stimuli.2,3 In addition, a variety of chemical compounds (e.g., histamine, bradykinin, serotonin, prostaglandins and, substance P) are released serially from damaged tissues and can activate or sensitize nociceptors.2,3 Serotonin has the additional action of modulating the peripheral release of primary afferent neuropeptides that are responsible for neurogenic inflammation. These neuropeptides include substance P, calcitonin gene-related peptide, and neurokinin A.2,3
Transmission involves the propagation of an electrical signal along neural membranes. Stimuli, such as prostaglandins and inflammatory mediators, change the permeability of the membrane, producing an influx of sodium and an efflux of potassium, thereby depolarizing neuronal membranes. Electrical impulses are transmitted to the spinal cord via two primary afferent nerve types: myelinated A-fibers and unmyelinated C-fibers. The A-delta fiber is responsible for rapidly conducting electrical impulses associated with thermal and mechanical stimuli to the dorsal horn of the spinal cord. A-delta fibers release excitatory amino acids, such as glutamate, which activate α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors located on dorsal horn neurons.2,4 Transmission of signals along these fibers results in sharp or stabbing sensations that alert the subject to an injury or insult to tissue. CNS input rapidly produces reflex signals, such as musculoskeletal withdrawal, to prevent further injury.
The smaller, unmyelinated C-fibers respond to mechanical, thermal, and chemical stimuli and conduct electrical impulses to the spinal cord at a much slower rate compared with myelinated A-delta fibers. C-fibers, which also terminate in the dorsal horn, release the excitatory amino acids, glutamate and aspartate. Unlike A-fibers, C-fibers also release peptides, such as substance P, neurokinin A, somatostatin, galanin, and calcitonin gene-related peptide (CGRP).5 The role of these peptides is not completely understood. Substance P is known to activate neurokinin-1 receptors, which may play a role in increasing excitability of spinal cord neurons.5,6 Transmission of electrical impulses via C-fibers results in pain that is dull, aching, burning, and poorly localized or diffuse. This type of pain is known as second pain because it is perceived after the first pain sensation.
Once dorsal horn receptors are activated, electrical signals are further propagated to the thalamus, primarily via the spinothalamic tract. From the thalamus, signals are sent to the cortex and other regions of the brain for processing and interpretation.
Modulation of nociceptive information occurs quickly between descending inhibitory pathways from the thalamus and brainstem and interneurons in the dorsal horn. Neurons from the thalamus and brainstem release inhibitory neurotransmitters, such as norepinephrine, serotonin, γ-aminobutyric acid (GABA), glycine, endorphins, and enkephalins, which block substance P and other excitatory neurotransmitter activity on primary afferent fibers.7
The conscious awareness, or perception, of pain is the end result of this complex cascade of actions. The perception of pain involves not only nociceptive processes, but also physiologic and emotional responses, which contribute significantly to the sensation that is ultimately experienced by the person.7 The perception of pain may be influenced by abnormal generation or processing of electrical pain signals and by the
psychological framework created by the patient's temporal affective state or from previous painful experiences. Therefore, treatment that includes drug therapy to alter the nociceptive and physiologic responses, in addition to cognitive-behavioral strategies (e.g., distraction, relaxation, and imagery) to alter the psychological response, may be more effective together than if either intervention is used alone.8
Peripheral and Central Sensitization
Under normal homeostatic conditions, a balance exists between excitatory and inhibitory neurotransmission. Changes in this balance may occur both peripherally and centrally, however, leading to exaggerated responses and sensitization.8,9 Examples often observed in chronic pain states include hyperalgesia (enhanced pain to a given noxious stimulus) and allodynia (pain in response to a normally non-noxious mechanical stimulus, such as light touch). Peripheral and central nociceptors can be functionally heterogeneous and can change through processes of sensitization. Peripherally, certain nociceptors respond to strong mechanical stimuli, whereas other normally “silent” nociceptors can undergo sensitization from exposure to prostaglandins, bradykinin, serotonin, histamine, adenosine triphosphate (ATP), and cytokines.10 These sensitized nociceptors then become highly responsive to weak mechanical stimuli.11
Centrally, two types of nociceptors have been identified. Nociceptive-specific neurons respond only to noxious stimuli, such as heat, whereas wide dynamic range neurons respond to noxious stimuli, but also may be excited by peripheral mechanostimulation.11,12 In chronic pain conditions, populations of these neurons can shift, so that normally inactivated neurons become highly responsive to various weak stimuli.2,11 The person affected notices that many types of stimuli elicit pain, including light touch or minor changes in ambient temperature.
On stimulation, AMPA receptors are the first to be activated, followed by neurokinin peptide receptors. The N-methyl-D-aspartate (NMDA) receptor does not participate in normal transmission and is blocked by magnesium.5,13 If the stimulus continues, NMDA receptors may become activated, leading to chronic painful conditions. For this to take place, several conditions must occur.5 First, input from primary afferent fibers must be of sufficient intensity and duration. When peripheral damage occurs, the synthesis and release of substance P is increased. The increased release of substance P, along with other peptides and neurokinins is thought to enhance glutamate activation of the NMDA receptor. Second, for glutamate to activate NMDA receptors, a coagonist, glycine, must be present. The last step in activating NMDA receptors requires the removal of the magnesium channel block. Tachykinins, such as substance P released along with glutamate, stimulate neurokinin receptors and depolarize the neuron. The magnesium block is removed only when there is sufficient repeated depolarization. When these conditions have been met, the receptor channel opens, allowing large amounts of calcium and sodium to enter the neuron. This produces excessive excitability and amplification of signals.
This initial activation of the NMDA receptor is known as wind-up. Wind-up progressively increases the number and response of nociceptive neurons in the dorsal horn without any change of input to the spinal cord.5,13 In fact, this process can continue after peripheral input has stopped. With continued nociceptive input, spread occurs activating nearby receptors, known collectively as metabotropic glutamate receptors.4,7 Central sensitization occurs when NMDA and neurokinin receptors are activated, along with increases of cyclic nucleotides and nitric oxide, and activation of several protein kinases within.11
Treatment Implications
If all these processes are considered, then the goal of pain therapy is to reduce peripheral sensitization, thereby decreasing central stimulation and the amplification associated with wind-up, spread, and central sensitization. This often requires multiple modalities to interrupt transmission at different levels. For example, the management of a chronic painful condition may include treatment with an opiate (e.g., morphine) to reduce ascending pain transmission, a nonsteroidal anti-inflammatory drug (NSAID) (e.g., ibuprofen) to reduce prostaglandin formation, and a membrane-stabilizing agent (e.g., carbamazepine) to alter ion flux in nerve membranes and blunt depolarization.
New targets will be identified for effective drug therapy as additional information is learned about the complex interactions and adaptations of neurotransmitters and receptors. However, it is unlikely that a single effective “magic bullet” will be developed because of the complex relationships between neurotransmitters and interpatient variability in the perception of pain. Such variability can arise from genotypic differences in receptor function, metabolic enzymes, and protein transporters that convey substances across cellular membranes. Therefore, continued understanding of the mechanisms of pain transmission will be important in allowing clinicians to most effectively use




0 comments:
Post a Comment